Creating a world where
Cancer Can’t Hide.
Cancer wins when it hides. Remaining undetected, cancer poses one of the greatest threats to life. When cancer wins, we all lose. But what if cancer couldn’t hide? What if there was a way to expose where it may be hiding and when it’s going to make its move?
Breast cancer has finally met its match.
Know where cancer may be hiding and when it might make its move.
Our Platform
ProFound Breast Health Suite
Backed by science, clinical evidence, and proven patient outcomes; our suite of solutions – Detection, Density, and Risk — shine a spotlight on cancer, exposing its hiding place. These clinically proven AI-powered solutions provide certainty and peace of mind, improving patient outcomes and saving more lives.
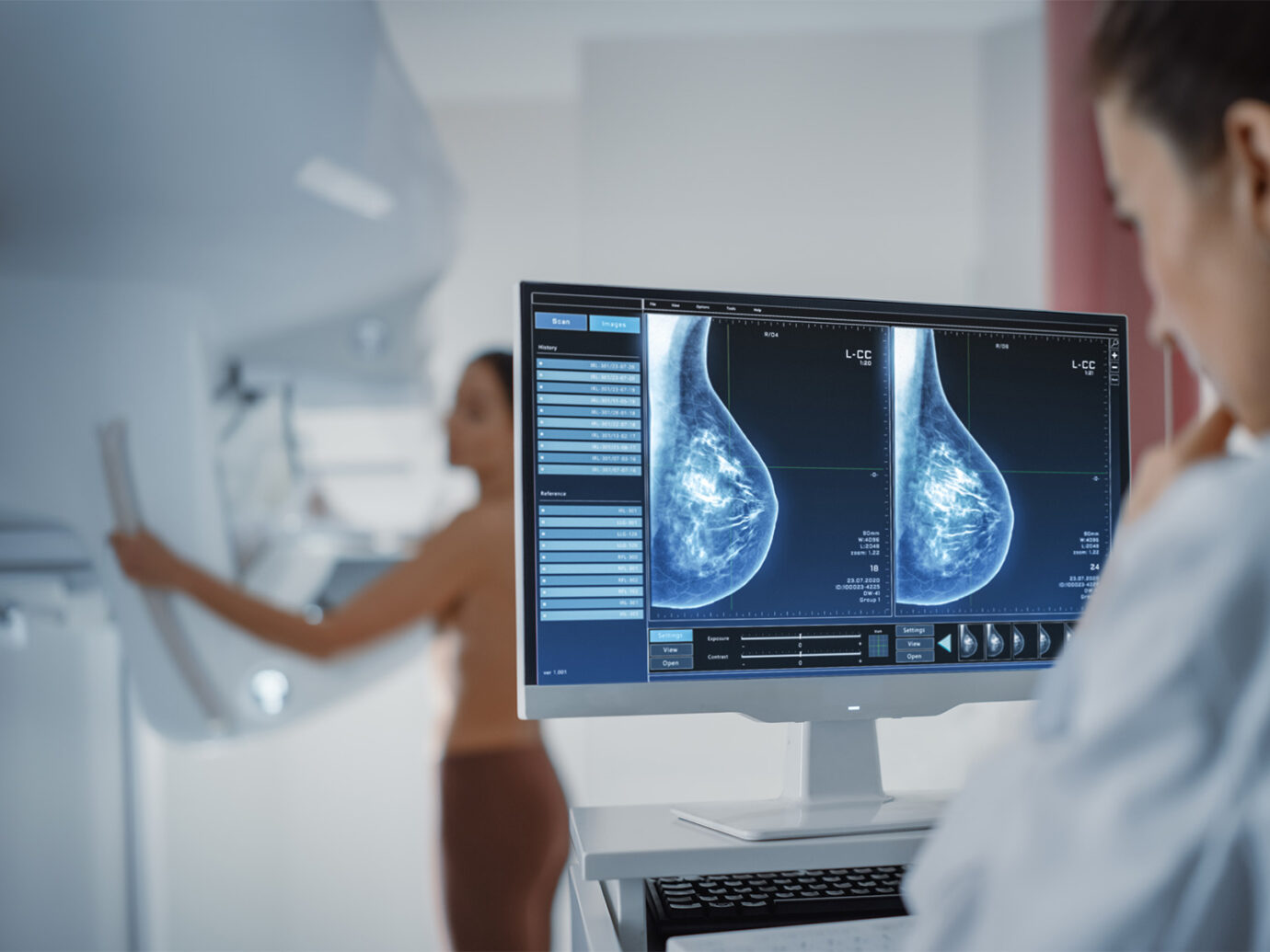
ProFound Detection
A screening revolution has arrived. The difference is ProFound.
Find cancers earlier, with greater accuracy and efficiency. With up to 2x enhanced clinical performance compared to other AI platforms,3 ProFound Detection offers unrivaled accuracy and workflow advantages.4
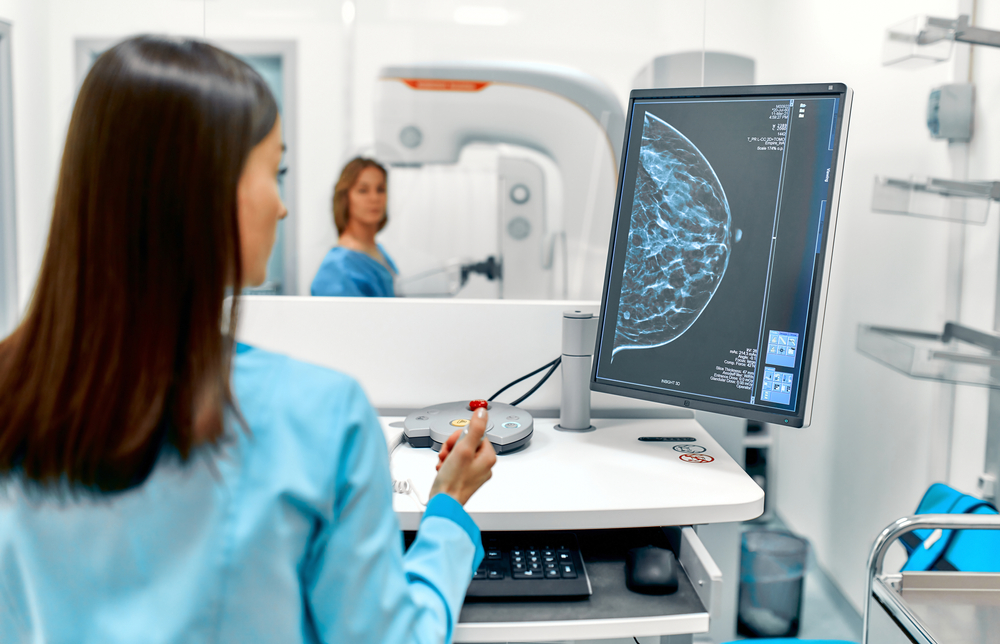
Density Assessment
Objective, reliable, consistent density scoring.
Breast density can elevate breast cancer risk up to 4x,6 therefore, density scores impact and inform personalized treatment plans. Simplify and standardize assessments of breast density, with results clinicians rely on and patients understand.

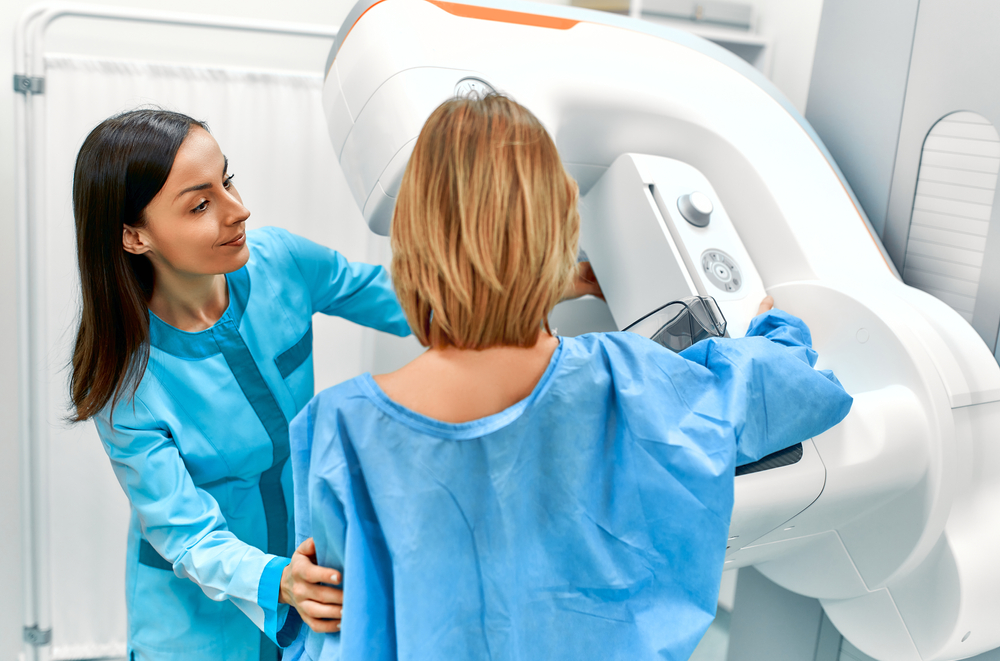
ProFound Risk
Know your risk. Protect your future.
The world’s first image-based risk model is 2.4x more accurate than traditional models.1,2 Identify women at high risk of developing cancer before or at their next screening to help find interval cancers, earlier.
Heart Health (Coming Soon)
Evaluate your heart health.
Breast cancer and heart disease are the top two leading causes of death among women. One mammogram can soon assess breast arterial calcifications, alerting you to heart concerns earlier.
About iCAD
Remaining unseen and insidious, hidden cancer poses one of the greatest threats to life. When cancer wins, we all lose. But what if cancer couldn’t hide? What if there was a way to expose where it may be hiding and when it’s going to make its move?
THE PROOF
Precise, powerful, proven
In the last five years alone, iCAD estimates reading more than 40 million mammograms worldwide, of which nearly 30% are tomosynthesis.
Expert Voices
Joining forces against a common enemy.
In the fight against cancer, we’re stronger together. Learn why our customers believe that our solutions are the secret weapon for cancer detection and treatment.
Our Partners
ProFound Partnerships
Proud to work with leading industry, research, technology, and advocacy partners.


Contact Us
Together, we can create a world where cancer can’t hide.
Ready to experience how ProFound can save lives?
1. Mikael Eriksson et al. A risk model for digital breast tomosynthesis to predict breast cancer and guide clinical care. Sci. Transl. Med. 14, eabn3971 (2022). DOI: 10.1126/scitranslmed.abn3971. 2. Eriksson M, Czene K, Strand F, Zackrisson S, Lindholm P, Lång K, Förnvik D, Sartor H, Mavaddat N, Easton D, Hall P. Identification of Women at High Risk of Breast Cancer Who Need Supplemental Screening. Radiology. 2020 Nov;297(2):327-333. doi: 10.1148/radiol.2020201620. Epub 2020 Sep 8. PMID: 32897160. 3. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Accessed 1-20-23. FDA 510K submissions K182373 (iCAD), K201019 (Hologic) and K193229 (ScreenPoint). 4. Conant EF, Toledano AY, Periaswamy S, Fotin SV, Go J, Boatsman JE, Hoffmeister JW. Improving Accuracy and Efficiency with Concurrent Use of Artificial Intelligence for Digital Breast Tomosynthesis. Radiol Artif Intell. 2019 Jul 31;1(4):e180096. doi: 10.1148/ryai.2019180096. PMID: 32076660; PMCID: PMC6677281. 5. https://www.nationalbreastcancer.org/early-detection-of-breast-cancer. Accessed 11-01-2023. 6. Lynge E, Vejborg I, Lillholm M, Nielsen M, Napolitano G, von Euler-Chelpin M. Breast density and risk of breast cancer. Int J Cancer. 2023 Mar 15;152(6):1150-1158. doi: 10.1002/ijc.34316. Epub 2022 Oct 27. PMID: 36214783; PMCID: PMC10091988.
iCAD data on file. Standalone performance varies by vendor.